2018
Immuron and US Department of Defense look to develop silver bullet for gastro infections
16 July 2018
US Defense finishes latest research on Immuron’s Travelan®
16 July 2018
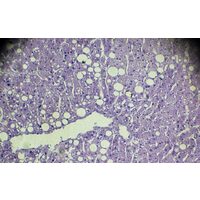
Immuron reports positive clinical study results in the fight against fatty liver disease
8 March 2018
Immuron's IMM-124E shows encouraging action in mid-stage NASH study; shares ahead 81%
8 March 2018
Immuron’s shares surge on demonstrating proof of concept for liver drug candidate
8 March 2018
Results in: IMC’s lead drug reduces major factor in liver inflammation
8 March 2018
Blockbuster Results from IMC’s NASH Liver Therapy Trials
8 March 2018